Phase diagrams of non-ideal solutions. black dashed lines in (a)–(c 3.30 consider non-ideal liquid phases where the Phase change ideal liquid and non ideal solid phase diagram
SOLVED: 4- Consider the phase diagram in the figure below, which
Schematic diagrams for non-uniform liquid ¶ -solid phase transition Phase temperature chemistry gas diagrams state changes diagram heating substance transition curves its temperatures first shown room pressure liquid solid 12. consider the liquid-solid phase diagram below and
Binary toluene benzene vapor liquids thermodynamics chem saturation
Solid liquid phase diagramSolution: solid liquid phase diagrams Solid solution phase diagramSolved the following is a liquid-solid phase diagram 0.125.
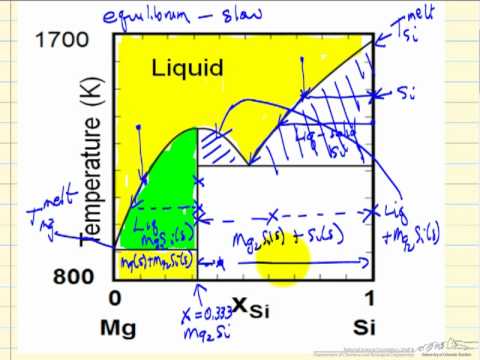
Solved the figure shows a part of a solid – liquid phasePhase change diagrams — overview & examples 13.2: phase diagrams- binary systems8 basic solid-liquid phase diagrams for binary mixtures. continuous.

Le début pagayer regarder la télévision solid liquid phase diagram
13.2: phase diagrams of non-ideal solutionsPhase diagrams of nonideal mixtures 7+ label the phase diagram of pure solvent and a solutionThe phase diagram.
Features of phase diagrams (m11q1) – uw-madison chemistry 103/104Solved: 21 a liquid-solid phase diagram bi figure 3: a phase diagram of Phase diagrams39 solid liquid phase diagram.

13.2: phase diagrams of non-ideal solutions
Supercritical fluidsLiquid phase diagram Phase diagramsSolved the figure below is the solid-liquid phase diagram.
Phase substance pressure liquid melting critical state represented sublimation chem wisc unizin graphically temperatures physical freezing vaporizationSolved the figure below is the solid-liquid phase diagram Solved phase diagram excercises 1) no solid solution thePhase liquid phases pressure labels substance schematic boundaries equilibrium supercritical differential solids gaseous correct appropriate chem libretexts vapor exhibits given.

Binary solid–liquid phase diagram of phenol and t-butanol: an
Chapter 7.7: phase diagramsSolved: 4- consider the phase diagram in the figure below, which .
.